Clinical Trial Design
SEVENFACT was evaluated to see whether it was effective and safe in the treatment of mild or moderate bleeding episodes in people with hemophilia A or B with inhibitors
This site is intended for U.S. residents only
SEVENFACT was evaluated to see whether it was effective and safe in the treatment of mild or moderate bleeding episodes in people with hemophilia A or B with inhibitors
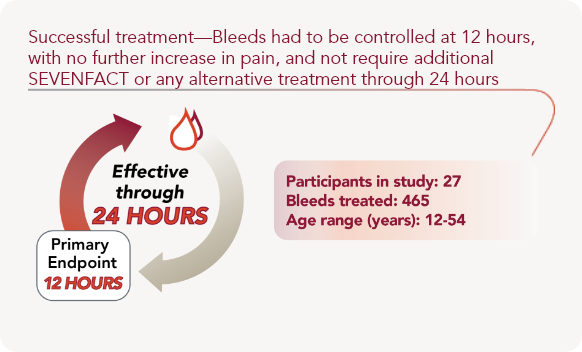
Proven to work across a range of inhibitor bleeds
A variety of bleeds were treated in the clinical trial
|
Joint |
|
|
Knee |
Shoulder |
|
Elbow |
Wrist/hand |
|
Ankle/foot |
Hip |
|
Soft Tissue/Muscle |
|
Nose |
|
Mouth |
|
Soft tissue/muscle |


The most serious possible side effect of SEVENFACT is abnormal clotting involving blockage of blood vessels, which include stroke, blockage of the main blood vessel to the lung, and deep vein blood clots.
You should know the signs of abnormal clotting and seek medical help immediately if they occur.
Signs of clotting in places other than your site of bleeding can include new onset of swelling and pain in limbs, new onset of chest pain, shortness of breath, loss of sensation or motor power, or altered consciousness or speech.
SEVENFACT is an injectable medicine used for the treatment and control of bleeding episodes occurring in adults and adolescents 12 years of age and older with Hemophilia A or B with inhibitors.
Injecting medicines requires special training; do not attempt to self-infuse unless you have been taught how by your healthcare provider.
You should not use SEVENFACT if you are allergic to rabbits, or if you have known allergies to SEVENFACT or any of its components. Seek immediate medical help if you experience hives, itching, rash, difficulty breathing with cough or wheezing, swelling around the mouth and throat, tightness of the chest, dizziness or fainting, or low blood pressure after taking SEVENFACT.
Tell your healthcare provider prior to using SEVENFACT if you have begun treatment of a bleeding episode with another bypassing agent.
Tell your healthcare provider if you are pregnant, are nursing, or plan to become pregnant; if you have had prior blood clots, heart disease or heart failure, abnormal heart rhythms, prior pulmonary clots, or heart surgery; or if you have or have had any other medical conditions.
The most common adverse reactions for SEVENFACT are headache, dizziness, infusion-site discomfort, infusion-site hematoma, and infusion-related reaction and fever.
Seek immediate medical help if you have signs of a blood clot or an allergic reaction.
To report SUSPECTED ADVERSE REACTIONS or product complaints, contact HEMA Biologics at 1-855-718-4362. You may also report SUSPECTED ADVERSE REACTIONS to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.