SEVENFACT 225 IDR*:
*IDR=initial dose regimen.
†225 mcg/kg initial dose regimen in the clinical trial.

|
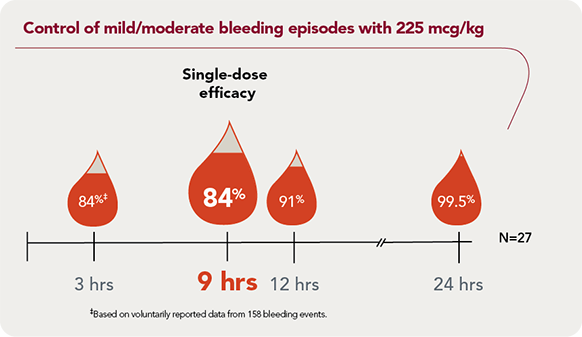
SEVENFACT 225 achieved control in 84% of mild/moderate bleeding episodes at 9 hours |

|
SEVENFACT 225—for mild/moderate bleeds, starting dose of 225 mcg/kg, followed, if necessary, 9 hours later by a 75 mcg/kg dose every 3 hours as needed |
|
SEVENFACT 225 IDR† |
|

|
Rapid effect: 3 hourAt 3 hours, 84% of mild/moderate bleeding episodes were controlled with a single dose§ |

|
Predictable response: 84%At 9 hours, 84% of mild/moderate bleeding episodes treated achieved bleed control after a single dose |

|
Reliable control: 99.5%At 24 hours, 99.5% of mild/moderate bleeding episodes were resolved |

|
Convenient home use: 98%98% of bleeding episodes were treated at home |
|
†225 mcg/kg initial dose regimen in the clinical trial. |
|
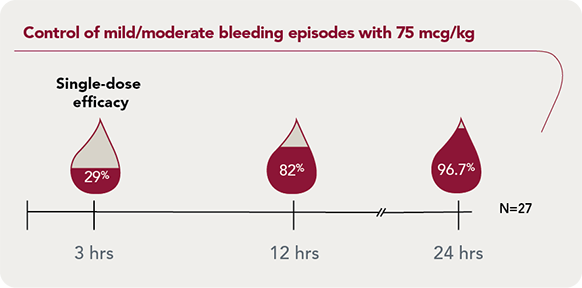
SEVENFACT 75 IDR: Dependable bleed control for mild/moderate bleeding episodes
SEVENFACT 75 IDR: Starting dose of 75 mcg/kg, followed by 75 mcg/kg every 3 hours as needed

|
96.7% of mild/moderate bleeding episodes treated with SEVENFACT 75 were resolved at 24 hours |
A proven safety profile
In the SEVENFACT clinical trial

|
No neutralizing antibodies |

|
No allergic reactions |

|
No thromboembolic events |
The most serious possible side effect of SEVENFACT is abnormal clotting.|| Seek immediate medical help if you have signs of a blood clot or an allergic reaction.¶
||What is the most important safety information I should know about SEVENFACT?
The most serious possible side effect of SEVENFACT is abnormal clotting involving blockage of blood vessels, which include stroke, blockage of the main blood vessel to the lung, and deep vein blood clots.
You should know the signs of abnormal clotting and seek medical help immediately if they occur.
Signs of clotting in places other than your site of bleeding can include new onset of swelling and pain in limbs, new onset of chest pain, shortness of breath, loss of sensation or motor power, or altered consciousness or speech.
¶Who should not use SEVENFACT (coagulation factor VIIa)?
You should not use SEVENFACT if you are allergic to rabbits, or if you have known allergies to SEVENFACT or any of its components. Seek immediate medical help if you experience hives, itching, rash, difficulty breathing with cough or wheezing, swelling around the mouth and throat, tightness of the chest, dizziness or fainting, or low blood pressure after taking SEVENFACT.
Adverse reaction occurrence was similar between the SEVENFACT 225 and SEVENFACT 75 dose regimens
Adverse reactions in SEVENFACT clinical trials (N=42)#
|
Adverse reaction |
Number |
|
Infusion-site discomfort |
4 |
|
Infusion-site bruising |
2 |
|
Dizziness |
2 |
|
Infusion-related reaction |
1 |
|
Headache |
1 |
|
Increased body temperature |
1 |
#In PERSEPT 1 and Phase 1b.

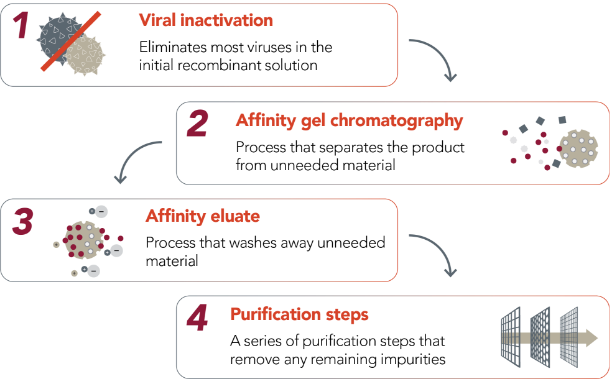
SEVENFACT purification and manufacturing
SEVENFACT is manufactured to high quality and purity for your safety and peace of mind.
SEVENFACT is a recombinant treatment for inhibitors and is not derived from human blood.

|
Goes through an extensive purification process before final production |
Predictable, reliable bleed control with just a SINGLE DOSE of SEVENFACT 225**LEARN MORE**As seen in the clinical trial. |

|
See what financial resources YOU may be eligible forFIND OUT MORE |


